MILKFAT PRODUCTS
Butter and other milkfat products are important economically to the NZ dairy industry and important nutritionally and functionally to customers. The range of milkfat products available has increased rapidly over the last two decades in response to competition from vegetable oil-based products. The traditional advantages for milkfat products over their competitors are flavour and dairy identity, and new manufacturing technologies now enable milkfat products to meet market needs for functionality as well. This article describes briefly the chemistry of milkfat and some of the processes used to manufacture milkfat products commercially.
Milkfat is mainly composed of the triglycerides of fatty acids, and occurs naturally in milk and cream as oil in water emulsions. In making butter from cream the phases must be inverted, as butter is a water in oil emulsion in which the oil phase is partly crystalline (“plastic”).. The two commercial butter making processes used in New Zealand are: Fritz, developed from the traditional batch churning process of crystallized cream, and Ammix in which fresh milk fat is mixed with cream and salt and shock cooled to give rapid crystallization.
INTRODUCTION
Fats are crucially important to the human diet, not just for their nutritive contributions (including energy, fat-soluble vitamins, and essential fatty acids) but because of their influence on mouthfeel and hence their ability to make much of our food palatable. In western cultures which have a surplus of dietary energy available, the textural and mouthfeel attributes imparted by fats to complex food systems become of paramount importance. Milkfat is particularly valued in these roles due to its flavour, which is almost universally accepted as desirable, and because it is perceived to be “clean and green” due to its “dairy identity”.
The chemistry of milkfat therefore spans a wide range, from the biochemistry of its production from grass to its interactions in complex food emulsions. The chemistry includes the melting and crystallisation of the major component triglycerides as well as that of the volatile flavour components which are present in trace amounts. Manufacturing conditions cause chemical changes to flavour and physical properties and these effects must be understood in order to control the functionality of milkfat in finished products.
Butter has played a substantial part in the export returns of New Zealand over many years and is still one of the main milkfat products exported from New Zealand. Though New Zealand’s butter production is not large by world standards, the major proportion is exported. In contrast, the major proportion of the butter production of other countries is consumed in their local markets. As a consequence, New Zealand has a major share of the world export trade in butter. In spite of the economic importance of butter and that it is regarded as a basic food item in many markets, consumers know little about the traditional butter making process, let alone the modern processing technologies which can produce a wide range of butter products tailored for specific end-use applications.
HISTORY OF BUTTERMAKING
Records show that the art of buttermaking dates back to very early times, the Indians of Asia using butter as a food prior to 2000 BC. Throughout history there are references to butter as a food and as a medicine and to its use in religious ceremonies. It was probably introduced into Europe by the Scandinavians, for whom it became an export item. There are reports that in the 12th century the Germans were sending cargoes of wine to Norway in exchange for butter and dried fish.
It was really not until the end of the 19th century that buttermaking left the farm, due mainly to the development of the centrifugal cream separator. This allowed cream separation to be carried out in central plants, processing large quantities of milk, and led to the mechanisation of buttermaking and large scale production.
DESCRIPTION
Butter is essentially an emulsion of serum (water, protein, lactose and salts) droplets dispersed in a continuous phase of semi-crystalline milkfat (water-in-oil emulsion). It has a subtle and desirable flavour, which, together with its “dairy identity”, gives milkfat and its products a commercial value far above that of most other natural fats and oils containing similar major components. The chemistry interests in milkfat are thus in the flavour compounds and how these are affected by processing conditions, and in the physico-chemical properties (e.g. crystallisation behaviour) of the milkfat itself and the emulsions in which it is almost always used.
Butter is the opposite emulsion from the milk and cream (oil-in-water emulsions) from which it is manufactured, and therefore in all buttermaking processes the stable cream emulsion must be inverted. The physical and chemical properties of the emulsion and its components (milkfat, serum and the interface between them) are critical to the control of the buttermaking process and have a major impact on economics.
Chemical composition
Before describing the key manufacturing processes, it is helpful to discuss briefly some of the properties of milkfat and its importance in buttermaking. Milkfat contains triglycerides (triacylglycerols) which consist of three fatty acids attached to a glycerol backbone (Figure 1). Over 500 different fatty acids have been found in milkfat, so a very large number of different triglycerides are possible. Hence milkfat is a complex mixture of triglycerides. Many of the triglycerides are present in very low concentrations, and over 200 have been isolated and identified. Figure 2 shows a typical gas chromatograph trace of milkfat triglycerides, separated simply on the basis of molecular weight.
Each peak contains triglycerides of the same carbon number (the sum of the number of carbons on the three fatty acids). For instance, triglyceride peak c50 contains three fatty acids with the carbon atoms
summing to 50, e.g. 16 + 16 + 18. The significant triglyceride carbon numbers present in milkfat range from c28 to c54. Table 1 shows the 11 most common fatty acids of milkfat that make up the triglycerides.
Because New Zealand dairying is based on efficient pasture production, there are seasonal changes in the milkfat characteristics that are influenced by the grass growth. This, together with the lactational effects of the cow, produces substantial changes in the composition of the milkfat. In the summer, there are more saturated fatty acids in the milkfat and this is reflected in harder butter. The unsaturated fatty acids and the short chain fatty acids contribute to softer fats and hence softer butter, such as occurs in the spring.
Minor components
The minor components are important to the nutrition and function of milkfat. The phospholipids provide emulsifying properties, $-carotene (Figure 3) provides the distinctive yellow colour of milkfat and there are also significant concentrations of the fat soluble vitamins such as vitamin A (retinol) and vitamin E (α-tocopherol).
Physical properties
The physical properties (such as hardness) of milkfat and its products are strongly influenced by the melting properties of the triglycerides, which in turn are dependent on the properties of their esterified fatty acids. The changes in the melting properties are reflected in the differential scanning calorimeter (DSC) trace (Figure 4). The DSC records the energy changes in a sample on heating at a constant rate, giving a direct measure of the apparent specific heat as a function of temperature.
Figure 4 shows the energy changes as a consequence of the melting characteristics (heat of fusion) of the milkfat. Milkfat melts over a very large temperature range, -40°C to 40°C, so most of the properties of milkfat relate to a semisolid, that is partially liquid and partially solid. The spring milkfat contains more low melting triglycerides (more melting occurring at low temperatures) and the summer milkfat contains more high melting triglycerides. Spring butter is thus softer than summer butter in New Zealand, but this is not necessarily true for dairy industries based on grain feeding.
In cream, approximately 99% of the fat is present as globules which typically range in size from 1 to 10 :m in diameter, with a mean of around 4 :m. Each of these globules is surrounded by a thin (less than 0.01 :m) protein-phospholipid membrane which primarily acts as an emulsifying system to stabilise the milk (or cream) emulsion, but it also protects the fat from enzymes which can break down the triglyceride structure. The composition of the globule membrane (and hence the stability of the emulsion and manufacturing properties) is affected by cow nutritional factors as well as processing operations in which shear (e.g. pumping), temperature cycling, aeration or dilution with water can occur.
Modification of properties
The melting properties of milkfat are seldom ideal for such diverse uses as a table spread which is spreadable straight from the refrigerator, or a plastic pastry fat designed for use at high ambient temperatures. Chemical modifications of the milkfat triglycerides or fatty acids, such as the hydrogenation and interesterification reactions used by the vegetable oil industry to modify fat melting properties, cannot be used because they would destroy the flavour compounds and the “dairy identity” essential to the commercial value of milkfat.
However, low temperature processes such as fractionation (fractional crystallisation) from the melt are used commercially to obtain milkfat fractions of different melting properties, while preserving the natural flavour and nutritional properties. Anhydrous milkfat is heated to above 40°C so as to be fully molten, and is then cooled slowly to crystallise out the high melting triglycerides, which are then separated to give a hard fraction (stearin) and a soft fraction (olein). The process can be repeated on the olein to give a series of milkfat fractions with widely differing melting properties.
Selected milkfat fractions can be blended for the manufacture of specialised butter products (using the scraped-surface heat exchanger (SSHE) technology described below) which can compete successfully on functional terms with vegetable-oil-based equivalents. The European dairy industry fractionates at least 100 000 tonnes of milkfat annually; although the equivalent New Zealand production is perhaps 10% of this, it is growing rapidly as export markets for the new products are developed. Anhydrous milkfat is a key ingredient in a wide range of recombined dairy products, including whole milk, cheese, spreads, ice cream etc., in countries that cannot support their own dairy industries and is thus vital to the New Zealand exporting dairy business.
BUTTER MAKING
In New Zealand, butter is made by two main processes: Ammix, based on SSHE technology, and Fritz, which was developed from the traditional cream churning process. Currently, more than half of New Zealand’s total butter manufacture is made by the Fritz process, but the Ammix process offers a number of advantages that are favouring SSHE technology increasingly for new plants. Although Fritz and Ammix butter making involve similar operations, the order in which they occur is different (Table 2).
Fritz butter making
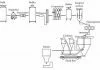
There are four main operations in the buttermaking process (Table 2 and Figure 5).
Concentration
The first operation is the concentration of the milk (4% fat), into cream of 40% fat using the centrifugal cream separator. The fat in cream is not free but is contained within globules which range in size from 1 to 10 :m diameter. Each globule is surrounded by a thin protein- phospholipid membrane which stabilises the globule as an oil-in-water emulsion. The cream is vacuum pasteurised and then steam-stripped to reduce some of the pastoral flavours. This flavour modification is very complex and the relationship of the chemistry to the sensory properties is still under investigation.
Crystallisation
The cream is then cooled to allow about 40-45% of the milkfat to crystallise to provide some rigidity to the globule for optimum buttermaking. If there is too much solid milkfat, the butter will be hard and brittle; if there is too little, then more fat will be lost in the buttermilk. The crystallisation is a slow process (usually left overnight) and the rate of cooling, the final temperature and the chemical composition are all important in determining the final texture.
Phase inversion
Fritz butter can be made by either batch or continuous processes. Microbiological problems with batch-churned butter and the trend towards larger butter plants caused a dramatic shift to continuous buttermaking in the 1970s, and now all of New Zealand’s butter is manufactured by continuous processes.
Table 2 — The main operations in butter making
The physical phase inversion occurs during the churning of the cream, which destabilises the fat phase of the cream. Under intense agitation with the incorporation of air, some of the membranes break, releasing fat which binds other globules, forming butter granules too large to remain in suspension. The fat thus separates and buttermilk (largely serum) is released and drained off, until a stable water-in-fat emulsion is obtained. This is a second concentration step to raise the fat content of the butter to the final level of about 80-84% fat.
Working (kneading)
The working (kneading) process, consisting of two rotating augers and mincing plates, works the granules into a pliable plastic mass and excess water is squeezed out, producing the butter texture. Before kneading, about 80% of the fat is still within globules, but this is reduced to 8-30% during working. The additional free fat released by this process helps provide the network structure that is characteristic of butter.
The final goal of the working is to give a homogeneous butter which is a finely dispersed water-in-oil emulsion. Salt can be added (as a slurry) and then further working disperses the water into small droplets of less than 10 :m. This fine moisture dispersion is important for product quality and shelf life. The final working is also under vacuum, which removes air and gives a smooth even texture. There are a variety of butter machines (comprising churning, separation and working sections) in use, with the largest ones capable of producing more than 10 000 kg of butter per hour.
The butter is then packed by automatic packing machines, either as consumer pats of various sizes (e.g. 113 g, 227 g etc.) or in bulk (25 kg blocks). The types of butter manufactured in New Zealand include salted, unsalted, whey (manufactured from milkfat recovered from cheese manufacture) and lactic (or cultured) butter.
The typical percentage compositions of salted and unsalted butter are given in Table 3.
Ammix buttermaking
The first step of Ammix buttermaking is the concentration of the milk to high fat cream (75% fat). High pressure homogenisation of the cream then causes the milkfat to coalesce and form a continuous butteroil phase in which the serum is dispersed as droplets.
Fresh milkfat is separated from the serum by centrifugation, and vacuum dried to produce fresh milkfat (MF) as shown in Figure 6 (PHE = plate heat ex-changer).
scraped-surface heat exchanger (SSHE) plant is then used to manufacture the Ammix butter. Cream, salt and fresh milkfat are then mixed in the appropriate proportions and shock cooled in a scraped-surface heat exchanger (crystallisation occurs in a matter of minutes rather than hours as required by the Fritz process) and worked and dispersed in a pinworker (Figure 7).
The mixing of individual ingredients means that the process is inherently more flexible than the Fritz process. The oil phase for Ammix buttermaking can contain milkfat itself, milkfat fractions of different melting points, and vegetable oils or hardstocks. Even anhydrous products (no serum phase) can be made.Variations of the Ammix process are used to make a wide range of functional milkfat products that cannot be made by the Fritz process.
These include spreadable butter (which spreads from the refrigerator), tropical butter (does not oil off at high ambient temperatures), low fat spreads, bakery ingredients (special butters for pastry and cake), and blends with vegetable oils, including fat mixes and blended table spreads.
This range of products is increasingly important for diversifying the branded products that help to insulate the New Zealand dairy industry from the fluctuations of international dairy commodity prices and thereby provide a more attractive environment for long term investment in the industry.
Manufacturing effects
The chemical composition is not the only factor affecting butter texture. One intriguing property of pure triglycerides is that they have multiple melting points, due to three distinct crystal forms (polymorphism). These different forms are found in milkfat to varying extents depending on the method of crystallisation, although, because there is a huge range of triglycerides, polymorphism is not the only important factor influencing milkfat properties.
Polymorphism is very important in cocoa butter used in chocolate production where blooming, a slightly grey surface with whitish spots, is a problem. Bloom is due to a transformation from the usual β1′-2 form to the large crystal β-3 form, producing lumps that protrude through the chocolate surface. Chocolate manufacturers expend considerable effort trying to stop or slow this transformation. Polymorphism is also important in margarine manufacture where there is less variety of triglycerides than in milkfat. The $’ form is preferred for spreadable products as the $ form produces large crystals which give a sandy mouthfeel to the products.
Because milkfat has many different triglycerides, they do not form pure crystals but tend to crystallise in groups of similar size and structure. So it is not just the polymorphic forms that are important but also the composition of these mixed crystals.
The presence of mixed crystals means that the melting points are different from those of the individual pure triglycerides, with a reduced overall melting range. The triglycerides within a mixed crystal are usually within 6 carbon numbers of each other. Three main groups can be distinguished in milkfat: a low melting group, an intermediate group and a high melting group as can be seen on the DSC trace (Figure 4). For milk fat, this mixed crystal formation is very important as the composition of the mixed crystals is affected by the cooling regime.
The faster the crystallisation, the greater is the variety of triglycerides that are crystallized together, and the harder is the product, because more low melting triglycerides are trapped within the crystal. In adition, the crystals tend to be small, 1-2 :m. If the crystallization is slow, there is more selectivity, and the crystals tend to be larger. This is important in butter making as crystallisation procedures can be changed during the dairy season to compensate for changes in the chemical composition of the milkfat and thus to reduce the variation in the texture of the butter.
With the importance of the crystallisation of the milkfat, it is not surprising that melting and recrystallisation under different conditions can produce different properties. As an example, butter that has melted on the picnic table and is then allowed to recrystallise when it is returned to the refrigerator is often harder than the original butter. This is due to changes to the structure resulting from the different crystallisation conditions.
CONCLUSION
The demand for exciting new food products in world consumer markets is driving product and process development at an ever-increasing pace. Understanding the chemistry of the transformations occurring as food ingredients are manufactured and used is becoming increasingly important for reducing development time and cost.
Even though the traditional butter making process appears relatively simple, there are many chemical interactions involved, both between milkfat components themselves and between the milkfat and other components in butter. When milkfat products are used as ingredients in sophisticated food emulsions the chemistry becomes even more complex, and the dairy industry is continuing to fund considerable research into this area. Thus in large measure, the profitability of functional milkfat products depends heavily on understanding all aspects of their chemistry.
EXPERIMENT
An entertaining experiment is to make butter in the kitchen mixer. The steps in the process outlined in Figure 5 of the Fritz process can be observed. Leave a carton of cream in the refrigerator overnight, to ensure that sufficient of the milkfat has crystallised, and then whisk using a mixer with a chilled bowl. When the granules are formed, they can be separated from the buttermilk with a kitchen sieve and washed with cool water.
Finally, knead with a rolling pin to make the butter pliable and to remove excess water. At this point, it is possible to add 1-2% salt (about 0.5-1.0% of the original cream weight) for flavour and keeping quality. As it is not possible to develop the fine moisture dispersion found in commercial butter, the keeping life of the butter made will be short.
Written by P A E Cant, K R Palfreyman, G D Boston and A K H MacGibbon (New Zealand Dairy Research Institute).
Comments
comments