The dairy industry handles large volumes of milk, and the major waste material from processing is the water. The water removed from the milk can contain considerable amounts of organic milk products and minerals. In addition cleaning of plant results in caustic wastewater. This article discusses the impact wastewater would have if released in the environment, methods to minimise the amount of both the organic and inorganic material in the wastewater, and methods of reducing the total volume of wastewater released. These methods involve improved techniques of recovering dissolved material and greater recycling of water.
The dairy industry like other industries in New Zealand has come under increasing pressure to improve its environmental performance. The pressures for change in New Zealand have come from changes in environmental legislation, trade negotiations and customers who are concerned about the conditions in which the product they are purchasing is manufactured. This article describes the effects of dairy processing operations on the environment , wastewater sources and characteristics, wastewater treatment, and air emissions .
ENVIRONMENTAL EFFECTS Effects on water The Organic Components The organic components of the wastewater from dairy processing operations can be classified as proteins, lactose and fat. These will affect the environment in different ways depending on their biodegradability and their solubility.
(a) River Oxygen Levels and BOD5 The organic components in dairy processing wastewater are highly biodegradable. In waterways, bacteria will consume the organic components of the waste. The process of biodegradation in waterways consumes oxygen according to the following equation:
Organic Material + O2 → CO2 + H2O + Bacteria
Measures of the amount of oxygen that are consumed by bacteria are the Biochemical oxygen demand (BOD5) and the chemical oxygen demand (COD). BOD5 is measured as the amount of oxygen that is consumed by bacteria in decomposing the waste over a 5 day period at 20o C. The COD is a measured by digesting the waste with boiling sulphuric acid and potassium dichromate in the presence of a catalyst, and the result is expressed as oxygen equivalents. In both cases the organic material is converted to carbon dioxide and water, but with the BOD5 test some of the organic matter is converted to new bacterial cells.
The concentration of oxygen in a river depends on both the rate at which oxygen is consumed by microorganisms and the rate of reaeration from the atmosphere. There are computer programs available to predict dissolved oxygen profiles. It is usual to perform a series of river surveys to obtain a best fit of the oxygen depletion/reaeration equations to the actual river conditions.
Oxygen is very important in rivers, primarily because it supports fish and other aquatic organisms. The usual lower limit for oxygen concentrations in rivers in New Zealand is usually about 6 g m-3. This level is based on the ability of sensitive fish species (usually trout and salmon) to survive.
Fully aerated rivers at temperatures of 15 to 25o C contain oxygen concentrations of at least 8 g m-3. It is therefore essential that discharges to rivers maintain an oxygen concentration of at least 6 g/m3 . In order for this to be the case the discharge to the river must not increase the river BOD5 by more than about 3 g m-3 (depending on the reaeration characteristics of the river).
(b) Sewage Fungus Low molecular weight organic compounds promote the growth of certain filamentous slimes in waterways. These bacterial colonies are collectively known as sewage fungus. The most common bacterial species in this category is Sphaerotilus natans.
In order to control sewage fungus, it is usual to place a limit on in-river BOD5 concentrations. Two limits have been suggested for use in New Zealand. These are that:
(i) the daily average soluble carbonaceous BOD5 concentration should be less than 2 g m-3, or
(ii) the daily average carbonaceous BOD5 of compounds with a molecular weight of less than 1000 daltons should be less than 1 g m-3.
One of the major constituents of dairy factory wastewaters is lactose, a low molecular weight sugar that is known to promote sewage fungus growth. Sewage fungus growth has been related to lactose concentrations in rivers by the equation:
Growth/g m-2 = 0.333 + 2.479 m(lactose)/g m-3
(c) Colour and Turbidity Wastewaters that are highly coloured are likely to alter the colour of a receiving water. Dairy factory wastes probably contain little soluble colour, although after various forms of treatment true colour may result.
Colloidal and particulate components in the waste reflect light back to an observer. This is known as apparent colour. The concept of turbidity is closely related to this phenomenon. Milk wastes contain significant quantities of material that will result in turbidity of discharges.
The Inorganic Components
(a) Nitrogen and Phosphorus One of the industry’s main aims is to recover the protein (organic nitrogen component) of the waste and convert it to saleable products. Nitrogen is, therefore, a very important component of the dairy factory waste waters. Some protein will be lost to the waste streams. Bacteria convert the nitrogen in proteins to the inorganic forms including ammonia, and the ammonium, nitrite and nitrate ions. Each of these inorganic forms of nitrogen have different environmental effects.
Nitrate ions are toxic in high concentrations to both humans and livestock. In young infants, nitrate can be converted to the nitrite form, absorbed into the bloodstream and convert haemoglobin to methaemoglobin. Methaemoglobin cannot transport oxygen. The condition of methaemoglobinaemia affects infants less than six months in age because they lack the necessary enzyme to reconvert the methaemoglobin back to haemoglobin. To protect humans the usual limit placed on drinking water supplies is 10 g m-3 of nitrate-nitrogen.
Livestock can also suffer from methaemoglobinaemia. Since ruminants have a more neutral stomach pH and rumen bacteria that reduce nitrates to nitrite, deaths from methaemoglobinaemia can occur. This usually results from the consumption of nitrate rich feed, although a limit of 30 g m-3 nitrate-nitrogen on drinking water for stock has been suggested.
Inorganic forms of nitrogen (nitrate, nitrite and ammonium ions) and inorganic phosphates act as plant nutrients in waterways. To protect receiving waters from “undesirable growths” it has been suggested that total inorganic nitrogen concentrations in receiving waters are limited to less than about 30-100 mg m-3 or that dissolved reactive phosphorus (inorganic phosphorus) concentrations are less than about 15-30 mg m-3.
(b) pH The pH of waterways are usually in the range 6 to 8. In order to protect aquatic life and, in order for water to be used by humans, it is necessary that the pH is not altered from this range.
(c) Temperature Most aquatic ecosystems are very sensitive to temperature. The temperature is also an important consideration when water supplies are to be used for drinking water purposes. It is usual to require that wastewater discharges will not alter the natural temperature of a waterway by more than 1-2 degrees.
Effects on land Wastewater application to soils is a common method of waste treatment in the dairy and other industries in New Zealand. This form of treatment is discussed in more detail in the section on wastewater treatment. Nutrients (nitrogen and phosphorus) The major mechanisms for nutrient removal in soil based treatment systems are:
- plant uptake and incorporation in animal products
- adsorption and immobilization in the soil
- losses to the atmosphere
- losses to groundwater (leaching)
Plant uptake of nitrogen amounts to up to 500 kg ha-1year-1. For phosphorus, the amount is about 30 kg of phosphorus. If animals subsequently consume the pasture, up to 90% of the nitrogen and phosphorus is recycled to the pasture.
Losses of nitrogen to the atmosphere occur through volatilization of ammonia from urine and dung patches, and through the process of denitrification. Denitrification is the process where microorganisms reduce nitrate to either nitrous oxide or dinitrogen gas. This occurs under anoxic conditions (i.e. a lack of oxygen) and when a suitable organic carbon supply is available for energy. Denitrification rates can be quite high at wastewater irrigation sites.
Losses of nitrogen (principally in the nitrate form) to groundwater can occur at some irrigation sites depending on the amounts of nitrogen removed by other means. The factor usually limiting the disposal of nitrogen containing wastes to soils is nitrate contamination of groundwaters that are subsequently used as water supplies for humans or stock. It is usual to apply normal drinking water guidelines under these circumstances.
Phosphorus does not usually cause a problem by leaching to groundwater because of the high retention and immobilization of phosphates in soils.
Sodium and Other Minerals
Sodium, potassium, calcium and magnesium are all immobilized by soils and occupy cation exchange sites on soil colloids and clays. The effects of this will be discussed later in this Chapter.
pH
Wastewaters with extreme pH values can affect soil pH values and, therefore, the microbiology, and nutrient availability in soils.
Effects on the atmosphere
Manufacturing operations can result in a number of emissions to the atmosphere.
Gaseous Emissions
Boiler stacks result in emissions of carbon dioxide, sulphur oxides and nitrogen oxides to the atmosphere. Methane may be emitted from anaerobic waste treatment systems and nitrous oxide (N2O) is emitted from the soil at wastewater irrigation sites. Carbon dioxide, methane and nitrous oxide are very important greenhouse gases, and it is likely that the consequences of these emissions will need to be considered in the future.
Dust/Odours
Particulate materials can be emitted from boiler stacks, powder driers etc . Losses of particulate material may also occur from other factory processes. If particulate emissions are high then surrounding buildings are coated with dust and powder which, as well as being undesirable, can also be corrosive.
The emission of objectionable odours must be considered at industrial processing sites. Many waste treatment plants can produce undesirable odours.
Visual Effects
The Resource Management Act requires that natural beauty is not degraded by the construction of buildings etc, especially in rural environments. Smoke and steam plumes from factories may also be regarded as a form of visual pollution. The height and size of the buildings is regulated by the District Council.
WASTEWATER SOURCES AND CHARACTERISTICS
Clean water
A variety of “clean” water discharges are produced by dairy processing operations. These include stormwater, cooling water, condensate (steam and evaporator), and permeates from membrane filters. These “clean” waters are usually contaminated to various degrees and may require treatment prior to discharge or reuse.
Water reuse
Problems with the disposal of wastewater have resulted in attempts to reduce the volume of wastewater and the components of the wastewater. At some sites, water is in short supply and the reuse of water is an attractive option.
The following strategy can be used to achieve the optimal water usage in a factory:
- Minimize the use of water in the present plant.
- Reuse water where possible without treating it first.
- Treat wastewater to allow its reuse.
- Optimize the use of reused water.
- Design or select new plant to use less water.
The reuse of water and chemicals is driven by economic and environmental considerations.
Water usage can be minimised by eliminating excess water use in product flushing, equipment startup and shut down, cleaning rinses, casein wash water and general running of hoses.
There are several areas in a manufacturing plant where both water recovered from milk and relatively clean water can be obtained for reuse. The requirement to treat the reused water will depend on the use of the water and whether the water will be in contact with product.
Water sources that have been considered for reuse are cooling water, final CIP rinse water, pump and separator seal water, condensate, casein wash water and membrane system permeates
Wastewater characteristics
The characteristics of the wastewater from manufacturing plants have changed as sites have become larger and byproducts and specific wastewater sources are separated for further processing, reuse or separate treatment and disposal. This has resulted in a wastewater with less organic matter in it as shown for individual processing plants in Table 1.
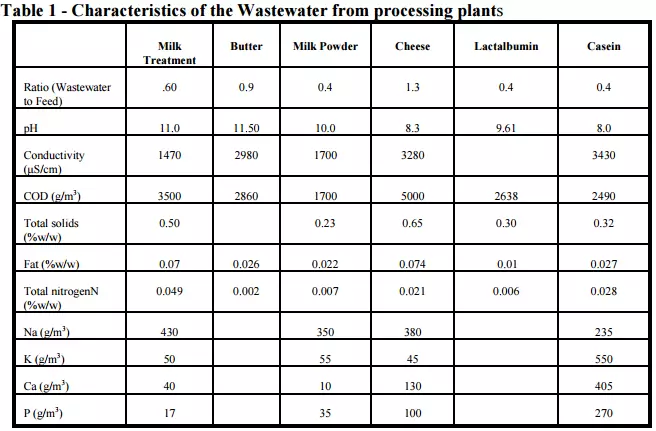
The wastewaters are typically characterized by the following:
- they are mainly diluted milk or milk products
- there are significant quantities of cleaning compounds and sanitizers present
- the contaminants are essentially organic with a high organic strength when compared to other waste streams like domestic wastes
- there are marked variations in hourly, daily and seasonal composition and flowrates which complicate their treatment
- they have a high BOD5 and COD
- they have a high sodium content from the use of caustic soda for cleaning.
Milk powder and butter plants tend to have a strongly alkaline wastewater while the production of lactic acid in the wastewater from cheese, casein and whey plants makes the wastewater from these plants acidic
Waste minimisation
Waste minimization is the:
1 Reduction in the generation of waste.
2 Reuse of waste materials/by-products.
3 Recycling of waste materials. Implied in this definition is a better use of the raw materials used in the processing plant.
The driving force for waste minimization for industry is improved yields of product, reduced effects on the environment and lower wastewater treatment costs. The New Zealand dairy industry has been committed to a waste minimization programme for the last 20 years.
Waste minimization programmes revolve around management and commitment to waste minimization/management. In the dairy industry, we have found the following strategy minimizes waste and improves yields:
- Obtain the commitment of management.
- Set up yield improvement team.
- Educate staff in yield awareness.
- Set goals to be achieved.
- Determine yield by measuring loss.
- Establish the cost of steps to improve yield.
- Take corrective action.
- Feed back results of the corrective action to management and staff.
Measurement of losses
A waste minimization programme in the dairy industry is governed by the loss monitoring programme. In the past, yields in the dairy industry have been calculated as:
![]()
This was changed to incorporate the losses determined by the loss monitoring technique.
Quantity of product packed = potential product in raw material – losses
Po = Pi – L
Direct measurement of losses, even with quite a low degree of accuracy, leads to an order of magnitude increase in the degree of the estimation of the yield. Areas which must be measured to determine losses are: wastewater, stack losses, product weights, product composition and stock food.
By-product utilization The byproducts in the dairy industry are now fewer in number. Skimmed milk from the production of cream and butter was at one time returned to the farms for animal feed but is now considered an integral product either as raw material for the manufacture of skimmed milk powder or casein for a number of new products.
Whey is the liquid left during cheese and casein manufacture after the protein curds have been removed from the milk. At one stage, all the whey produced from cheese and casein manufacture was used as animal feed, applied to land as a fertilizer or required disposal. Today’s technology allows a wide range of products to be manufactured from whey. Removal of water by evaporation produces concentrates for use as whey cheese, or recovery of lactose for incorporation into other foods.
Ultra filtration/ion exchange processes allow the whey to be separated into its components, protein, lactose, and minerals. The current whey processing technology is very capital and energy intensive and, while at present market returns must be able to finance the operation before a whey processing facility is built, the situation is changing where the cost of wastewater treatment is also being considered.
Chemical usage
The chemical usage during the cleaning of the plant is also a very important factor in waste minimization and economics. Extensive work is carried out on all plants to ensure only as much chemical as required is used in each cleaning cycle. Strengths of cleaning solutions, timing of rinses and microbiological testing of the first product through the plant help to minimize chemical usage but also ensure the plant is hygienically clean.
milkfat processed milkfat in product Yield = III-Dairy-J-Environment-8 Some plants have a reuse Clean In Place (CIP) system. Reuse CIP systems can take many forms from saving the sodium hydroxide solution to use as an initial clean on the next plant CIP followed by a clean using new sodium hydroxide solution to diverting the alkali or acid to a reuse tank which may be used for one or two weeks.
The life of the sodium hydroxide solution can be extended by draining the sludge which settles to the bottom off, leaving the “good” sodium hydroxide in the tank, or alternatively decanting the good sodium hydroxide solution from the settled sludge. The strength of the sodium hydroxide and microbiological quality are monitored regularly and, at present, the systems are only used once the processing plant has been initially well rinsed with water which may lead to false savings as more water is used. Conductivity measurements are used to detect the interface between rinse water and cleaning chemicals
Membrane technologies have been used within the dairy and other industries to recover specific products. Dairy Companies are now beginning to use them to recover cleaning chemicals for reuse. A current research project is investigating the use of both microfiltration and nanofiltration to recover caustic based cleaning agents and nitric acid. Between five and ten reuses of the caustic based cleaner were achieved on an evaporator and nitric acid was used approximately three times as long as previously before being discarded.
Cost benefits of improved yields and reduced losses
For a site processing 2 000 000 L of wholemilk/day, a loss of 0.5% of the raw material results in a loss of 1350 kg milkfat equivalents/day. If this is rectified, then considerable extra revenue would result. There could also be an additional benefit for waste treatment operations. The increase in yield will result in a reduction of 1040 kg BOD5 to treat with savings in treatment costs.
Waste minimisation measurement
To measure the losses from a dairy plant the quantity of material in the wastewater must be determined. This is obtained by measuring the volume of wastewater and then sampling and analysing the wastewater.
Wastewater Measurement
Flumes and weirs are structures placed in gravity drains that allow the flowrate to be measured by measuring the height of liquid at a set position upstream of the structure. Representative samples of the wastewater are also taken and analysed for fat, protein, organic matter and cleaning chemicals.
At some sites in New Zealand in-line meters are used to give an indication of the losses to the wastewater. These include turbidity meters, conductivity meters and pH meters.
WASTEWATER TREATMENT
As described previously, dairy processing wastewaters contain substantial quantities of organic matter, nitrogen and phosphorus. If excessive concentrations of these enter waterways, oxygen depletion and plant growth in the waterways may reach nuisance proportions.
The manufacturing dairy industry uses two main methods of treating wastewater: biological treatment in extended aeration systems and by spray irrigation to pasture. Currently, six sites use biological treatment while 17 use spray irrigation. Table 2 shows the main methods by which the New Zealand Dairy Industry treats the wastewater generated from the manufacturing plant.
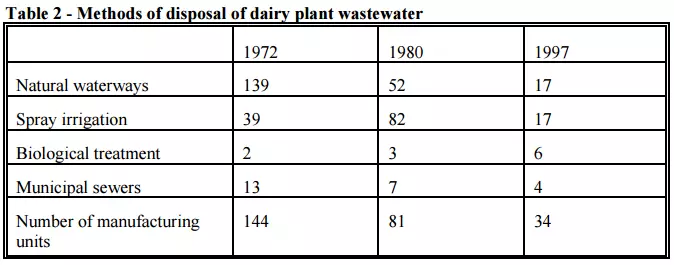
Pretreatment
Pretreatment in the dairy industry for many years meant some form of dampening flow, pH or organic load variations and a rudimentary fat/solids tank. This is changing with the industry now installing pretreatment systems to reduce loadings on wastewater treatment systems and also to allow some factories to continue to discharge to municipal systems. Pretreatment systems are now being maximised to remove solid material using air flotation principles coupled with neutralisation of the wastewater and the addition of flocculants and polyelectrolytes.
These systems, while removing solids and nutrients, are limited in their ability to reduce the organic loading in the wastewater because the main source of BOD5 in dairy plant wastewater is lactose which is soluble and hence cannot be removed by physical/chemical means.
The disposal of the recovered material can be of concern as environmental pressures increase and the solid material can not safely be placed in landfills as it is still biologically active. Biologically active solids can be composted and utilised as a fertiliser (Van Oostrom and Cooper 1988). Work is being undertaken in New Zealand whereby the solid material is heated, the fat used in other processing industries and the remaining material, mainly protein, is being composted.
Land treatment
Land treatment systems are used extensively in the New Zealand dairy industry. They use the soil as a biological medium to treat the components of the applied wastewater and hence they need to be designed to the appropriate criteria to ensure efficient operation.
Organic Loading
When wastewater is applied to pasture, soil microorganisms convert the organic matter present to carbon dioxide and water. During this process, biological slimes and additional bacteria are produced.
On fine textured soils the production of slimes etc can inhibit the movement of liquid through soil pores and lead to undesirable effects such as ponding. Dairy factory wastewaters can contain high concentrations of BOD5 primarily due to their lactose, fat and protein content. In the soil matrix, the normally soluble lactose is converted to bacteria. Research has shown (e.g. Parkin and Marshall, 1976) that an organic load of 2000 kg BOD5 per hectare is utilized over 16 to 20 days. Higher loadings can be used on some free draining soils. This application rate represents a design load of about 250 m3 per hectare on each irrigation occasion.
Sodium Ion Concentration
Excess applications of sodium ions without sufficient amounts of the divalent calcium and magnesium ions can lead to swelling of clay minerals and deflocculation of soil colloids. The long term consequence of this is a much reduced soil infiltration.
The chemical processes that occur are difficult and costly to reverse. The critical wastewater parameter related to minimizing sodium ion effects is the amount of sodium present relative to the concentration of other, especially divalent, ions present. Various analytical ratios have been used to monitor these effects. One of these is the Wilcox (Wilcox, 1948) ratio which is defined as:
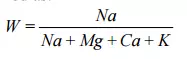
The critical value for the Wilcox ratio is 0.8 where the units are milliequivalents per litre, (mol L-1 for monovalent cations and 0.5 mol L-1 for divalent cations). The values of the Wilcox ratio calculated from the data in Table 4.2.1 are 0.62 for casein/cheese wastewater, 0.97 for the milk powder/butter waste, and 0.20 for whey. The amount of sodium relative to total cations is relatively high for the wastewaters compared to the whey because of the use of caustic cleaning chemicals in the factories. This is most pronounced for the milkpowder/butter wastewater, and it is necessary to add calcium to this system. This has been achieved at some sites by applying lime to the soil. It is also possible to add a calcium salt to the wastewater prior to irrigation.
Another parameter that is commonly used to determine whether or not the amount of sodium in a wastewater is excessive is the sodium adsorption ratio or SAR (Ferguson, 1976). This concept is based in equilibrium theory and is defined as:
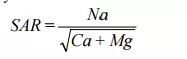
where the concentrations of the metal ions are also expressed as millimoles per litre. The SAR for casein/cheese, milk powder/butter and whey wastes are 9.6, 49.6 and 4.4, respectively.
The critical value for the SAR depends on the soil type but is generally accepted to be about 15. Once again, this parameter shows that irrigation of milkpowder/butter wastewaters requires the addition of a suitable calcium source to either the soil or the wastewater. A wastewater characteristic related to its cation content is its ash content.
It is usual to keep the ash content of irrigated wastewater to below 0.2% (w/w) to prevent a buildup of minerals in the soil and possible pasture growth problems.
Nutrient Loading
(a) Nitrogen
When wastewaters are applied to soils, microorganisms mineralize organic forms of nitrogen. Organic nitrogen is converted to the ammonium and then nitrate forms which can be directly utilized by plants. Nitrate can also be lost to the atmosphere by the process of denitrification, or can leach to the groundwater.
At a hydraulic load of 250 m3 per application and a 20 day return cycle the data in Table 3 shows that the average annual nitrogen loading when a casein/cheese wastewater is irrigated is 500 kg ha-1. For a milk powder/butter wastewater the nitrogen loading is 175 kg ha-1
The amount of nitrogen removed at an irrigation site depends on a number of factors, including the amount of nitrogen applied, the soil types, the moisture regime at the site, whether or not the area is cropped or grazed, and the management practises. Most dairy plant irrigation sites are operated as productive dairy farms.
These farms produce about 11200 L milk/ha annually, resulting in a net nitrogen removal in milk of 63 kg per hectare each year. An additional 10-12 kg ha-1 is deposited in body protein and replacement stock. The process of denitrification is another important mechanism resulting in the removal of nitrogen to the atmosphere and reduced losses to ground water. Denitrification losses are difficult to predict and also depend on soil types, moisture regimes at the site, groundwater conditions and management practices.
Total denitrification losses measured at wastewater site are in the range 200-250 kg ha-1 year-1. Typical mass balances for the irrigation of casein/cheese and a milkpowder/butter wastewater are shown in Table 3 nitrogen loses of the magnitude indicated are in the range accepted by many Regional Councils. The New Zealand Dairy Research Institute has monitored groundwater at several wastewater irrigation sites in New Zealand. Although the amount of nitrate nitrogen in groundwater under and near dairy factory irrigation sites is highly variable, at many sites the concentration is about or less than the World Health Organization recommendation of 10 g m-3.
(b) Phosphorus
Phosphorus leaching at wastewater irrigation sites is not usually a problem in most New Zealand soils because either the phosphorus loading on the soil is limited by the type of wastewater irrigated or the processes that immobilize phosphorus in soils are very active. Phosphates can be fixed by silicate clays, take part in anion exchange reactions, incorporated into organic matter and precipitated by iron, aluminium, manganese and calcium salts.Phosphorus is therefore generally retained in the soil profile.
Soil characteristics
When considering soils that are suitable for irrigation of wastewaters it is necessary to consider:
- drainage
- infiltration
- cation
- exchange
- capacity
- anion exchange capacity
- clay mineralogy.
Hydraulic Loading
Soils must be free-draining and have good infiltration capacities. Dairy factory irrigation schemes are designed with an application rate of 6 mm/h. They are also designed to apply 250 m3 /ha/dose although this may be increased on good volcanic soils. The method of wastewater application adopted and found most successful by the dairy industry is the fixed underground network system. Border-dyke systems are used in other industries (e.g. the meat industry) but loadings are generally higher at the top end of the borders, and clogging of the soil pores is common.
Effects on farm production One of the major benefits of wastewater irrigation is that an economic return through farm production is obtained. Many alternative forms of treatment (e.g. extended aeration) do not recover nutrients from the wastewater but result in their discharge to receiving waters.
As well as increasing total farm dry matter production, wastewater irrigation can affect the composition of the pasture. The results from taking eight samples over a two year study period at a site receiving a milk powder/butter wastewater show that the phosphorus, sodium and sulphur content of the pasture are all elevated by wastewater irrigation.
Biological treatment
The dairy industry uses aerobic or anaerobic treatment, or a combination of both, to treat the wastewater. Aerobic systems require an energy source to provide the oxygen required to assimilate the organic matter in the wastewater and hence are more suited to low to moderate strength wastewaters, since the higher the organic content the greater the oxygen demand and the greater the costs. Anaerobic systems have been developed for their ability to treat high strength wastes and the utilization of the methane gas.
Aerobic systems
In aerobic treatment systems, bacteria, in the presence of oxygen, convert the organic components of the waste to carbon dioxide,water and bacterial biomass. All aerobic treatment systems have the potential to cause odours if operated incorrectly. The industry worldwide has tried many forms of aerobic treatment.
These have included trickling filters, rotating biological contactors and various forms of mechanically aerated lagoon systems.
In New Zealand only extended aeration activated sludge systems are used. Typical treatment parameters for an activated sludge plant treating dairy plant wastewater are 94% COD, 99% BOD5 70% TKN and 50% total phosphorus removal.
Anaerobic treatment
Considerable experimental work has been undertaken on the anaerobic digestion of whey from casein and cheese plants (Clark,1988: Switzenbaum and Danskin, 1982). Various forms of high rate anaerobic digestion systems have been investigated with whey. However, few anaerobic systems treating whey have been installed, despite such systems being operationally viable and the value of methane produced from these systems as the industry values the components of the whey more highly. In an anaerobic digester, anaerobic bacteria, acting in the absence of oxygen, convert the organic components in the wastewater to methane, carbon dioxide and water. Organic forms of nitrogen are converted to the ammonium nitrogen form.
Anaerobic digestion may be carried out in low rate lagoon systems or in high rate reactors. The more recent anaerobic digesters which have been installed in the dairy industry have been high rate digesters, usually with two stages to obtain better control of the anaerobic processes.
The advantages of anaerobic digestion are: –
produce a valuable byproduct (methane), that can be recovered and utilized as a fuel – remove substantial quantities of BOD5 and COD without the input of mechanical energy for aeration – produce less sludge than aerobic systems
Nutrient removal
Dairy factory wastewaters contain substantial quantities of the plant nutrients nitrogen and phosphorus. If excessive concentrations of these enter waterways then they will promote the growth of plants in the waterways. Eventually these may grow to nuisance proportions. In New Zealand, wastewaters from dairy manufacturing are usually treated in either extended aeration activated sludge plants and discharged to suitable waterways, or are irrigated onto land after primary treatment.
Activated sludge systems can remove some of the nitrogen and phosphorus in the waste sludge because these same nutrients are also required for bacterial growth. However, overall removals will, in some cases, be insufficient to meet environmental demands. Under these circumstances an alternative form of treatment, or an add-on to the existing treatment will be required to meet discharge requirements.
Chemical Methods Chemical methods of phosphorus removal utilize the low solubility of metal phosphates. Both ferric and aluminium phosphates show minimum solubility between pH 5 and 6. A variety of different calcium phosphates exist and these show minimum solubility at high pH values (usually greater than 9). Reports exist in the literature on the use of iron (both ferrous and ferric ions), aluminium and calcium salts for chemical phosphate precipitation. It has been found that about twice the molar ratio of metal ion to phosphorus is required for effective phosphate precipitation.
Research conducted by NZDRI suggests that with milk processing wastes even higher metal to phosphate ratios are required, and that calcium ions are not very effective at removing phosphates. It is assumed that this is in some way related to the proteins that are present in milk wastes and that they compete with phosphates for bonding to the metal ions.
Typical treatment efficiencies for phosphate precipitation using ferric, ferrous and aluminium salts results in phosphorus concentrations in the effluent of less than 1 g m-3. Treatment to this degree will result in insufficient phosphorus being available for subsequent biological treatment. Chemical precipitation of phosphorus will be most effective after biological treatment. Biological Removal Bacteria require phosphorus for growth and biomass contains about 2.6% phosphorus.
Therefore, biological treatment systems that produce excess biomass, such as the activated sludge process, will remove some phosphorus from wastewaters. For an excess sludge production rate of 20% of the BOD5 consumed the activated sludge process will remove about 50 g/m3 phosphorus from a cheese/casein wastewater and 9 g/m3 from a milk powder/butter plant wastewater. These removals represent about 70 and 25%, respectively, of the phosphorus initially present in the wastewaters.
It has been found that biological phosphorus removal can be enhanced by using alternating anaerobic and aerobic conditions in the treatment system. During the anaerobic phase low molecular weight organic acids are absorbed by the microorganisms. The energy required for the absorption process is made available by the release of phosphorus bound as polyphosphates in the bacteria.
When the bacteria are subsequently exposed to aerobic conditions the organic matter is metabolized, energy is made available for growth and reaccumulation of phosphates occurs. During the aerobic phase the bacteria contain 3 to 6% phosphorus. Removal of excess microorganisms from the treatment process results in enhanced phosphorus removal. REDUCTION OF AIR EMISSIONS The main emissions from boiler stacks are nitrogen oxides, sulphur oxides and particulates (ash and small quantities of solid fuel). Driers are extemnwsively used by the dairy industry to dry a wide variety of milk powder products.
The main methods used to reduce atmospheric emissions in the dairy industry are:
(i) Cyclones, and Multicyclones III-Dairy-J-Environment-16 Cyclones impart a swirl to combustion gasses, and separate heavier particles from the outside portion of the gas stream. These units are effective for larger particles.
(ii) Baghouse Filters Bag-filters separate fine particles. Large surface areas are required.
(iii) Electrostatic Precipitators. Strong electrostatic fields result in particles acquiring electric charges, and being attracted to, and precipitated on, large plate electrodes.
(iv) Wet Scrubbers Flue gas passes upwards through a chamber while water (with or without various additives) is sprayed down through the chamber, absorbing contaminants.