CHEMICAL ANALYSIS IN THE NEW ZEALAND DAIRY INDUSTRY
Any dairy product is a variable mixture of a large number of different chemical species, which are usually grouped together into broad classes such as moisture, fat, protein (defined as (protein nitrogen (N) + non-protein nitrogen (NPN)) ! 6.38), anhydrous lactose and mineral salts (which includes citrate) for proximate analysis. If all the chemical species present in their native states are designated to a particular class, we would expect the total of the individual classes to add to 100%. However, when the individual classes are analysed and totalled together, they seldom add to exactly 100%. This is due to the experimental errors in carrying out the test and the known systematic errors in the determination of most components which are particularly important in the case of mineral salts (usually determined as ash) and protein. Also, some of the systematic errors are often partially compensating; for example, in the analyses of the solids of milk, the overestimation in protein (approximately 0.1%) because the NPN fraction is multiplied by 6.38 instead of the more correct figure of 3.60 is approximately compensated for by the underestimation of the mineral salts as ash. Also, it must be noted that, with the exception of water and perhaps lactose (where the lactose molecule is measured precisely by enzymatic, gas-liquid chromatography or high performance liquid chromatography procedures), the major dairy classes cannot be measured directly, but are inferred indirectly using carefully standardised analytical methods that embody specific assumptions about the chemical properties of each class. This factor adds further uncertainty in being able to account for exactly 100% of the dairy product when summing the major classes.
INTRODUCTION
Chemical analysis in the dairy industry ranges from the traditional to the modern, from the simple to the complex. An example of a traditional method (described later) which is of major importance to the industry is the Kjeldahl method of nitrogen determination which is used to ascertain the protein content of dairy products. Whereas one of the latest techniques being used to identify the various peptides produced from the hydrolysis of milk proteins is LC-MS-MS, which is liquid chromatography – mass spectrometry – mass spectrometry, where the tandem mass spectrometer is in reality a sophisticated detector for the liquid chromatography instrument.
An example of a relatively simple analysis would be the determination of moisture however this assay still requires skill to obtain good precision. In contrast, several of the vitamin assays are reasonably complex and can take up to 2 days to obtain a result. Most testing involves gross compositional analysis for the determination of protein, fat, moisture, lactose, total solids and ash. Other testing involves macro elemental analysis for calcium, magnesium, sodium, potassium, phosphate, nitrate and nitrite.
Another area of testing looks at the levels of trace elements, vitamins and other natural minor components such as fatty acids and cholesterol. It is probably fair to say that analysis in the dairy industry has undergone a revolution in the past decade and has effectively come of age. The need for experienced analysts has never been more acute as to be proficient one has to be involved in traditional “wet” chemistry, GC, HPLC, infra-red, atomic absorption, auto-analysers, electrophoresis, data processing packages, spread sheets, statistics, etc, etc. So why do we need to perform all these tests?
There are several reasons:-
(i) Truth of labelling – basically being able to assay what is claimed on the label. For example the additional vitamins and minerals added to milk powders for use as baby foods or the additional calcium in calcium fortified skim milk.
(ii) Food safety. Certain unnatural compounds present in milk products that arise during processing are required to be less than certain levels, eg, nitrates and nitrites.
(iii) Compliance with the NZ Food Regulations. For example butter is required to have less than 16% moisture.
(iv) Farmer payment. This is to ensure that there is a fair and equitable method to pay farmers for their milk.
(v) Establishing a standard milk composition. This provides the NZ Dairy Board with a basis to pay industry for specific products based on a standard milk composition.
(vi) To measure the main components of the milk as it enters the factory. As product processing is highly dependent on milk composition accurate and rapid methods of measurement are required.
(vii) To provide the necessary information required to genetically improve the national herd. Different breeds of cows provide different amounts of fat and protein as do different genotypes of the same breed. Many of the routine chemical tests are performed by rapid automated instruments, such as the “milkoscans”, which have been calibrated for protein, fat, lactose, moisture and total solids determined by reference methods. In effect these instruments provide the basis for secondary reference methods.
The composition of milk
Milk is defined by the New Zealand Food Regulations, 1984: “Milk or raw milk shall be the normal clean and fresh secretion obtained by emptying the udder of a healthy animal that is properly fed and kept, excluding that obtained during the four days immediately following the birth of its offspring.”
REFERENCE TESTING OF MAJOR MILK COMPONENTS
Gravimetric Fat Determination Fats are generally considered to be esters of glycerol with various carboxylic acids, and in most cases what is meant by fat is triglyceride.
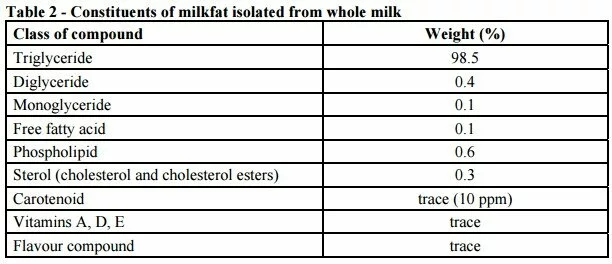
 The number of free OH groups determines whether the fat is a triglyceride, diglyceride or monoglyceride. Generally, the term used for dairy lipid is milkfat because most of the fat (over 98%) from milk is triglyceride. However, milkfat contains other minor lipid compounds. In some dairy products, these minor components become major components, e.g. products with high levels of phospholipids.
The number of free OH groups determines whether the fat is a triglyceride, diglyceride or monoglyceride. Generally, the term used for dairy lipid is milkfat because most of the fat (over 98%) from milk is triglyceride. However, milkfat contains other minor lipid compounds. In some dairy products, these minor components become major components, e.g. products with high levels of phospholipids.
The two important major factors are as follows: –
Triglyceride is the major fat of milkfat (99%). – Triglycerides are non-polar hydrophobic (water hating) compounds. These factors have important implications in the way milkfat tests for milk have been designed. The 2 reference test procedures, the Rˆse-Gottlieb and the Schmid-Bondynski-Ratzlaff methods, are extremely efficient in extracting triglycerides and other non-polar fat compounds but they vary considerably in the efficiency of extraction of other more polar fat compounds (e.g. free fatty acids, monoglycerides and phospholipids).
This means that the fat tests are particularly efficient in measuring milkfat from milk consisting of 99% triglyceride, but are not particularly efficient in measuring fat from products that have a high percentage of polar lipids such as phospholipids or free fatty acids.
As milkfat consists of a diverse range of organic compounds with varying physical properties which makes it difficult to give milkfat a ìwater-tightî definition that applies to all fat components. Several definitions can be used.
However the Nutrition Labelling and Education Act of 1990 in the USA has mandated a chemical definition for total fat as:“Total lipid fatty acids, that is the sum of fatty acids from mono, di and triglycerides, free fatty acids, phospholipid fatty acids and sterol fatty acids to be expressed as the amount of triglycerides that would provide the analytically measured amount of total lipid in the food.”
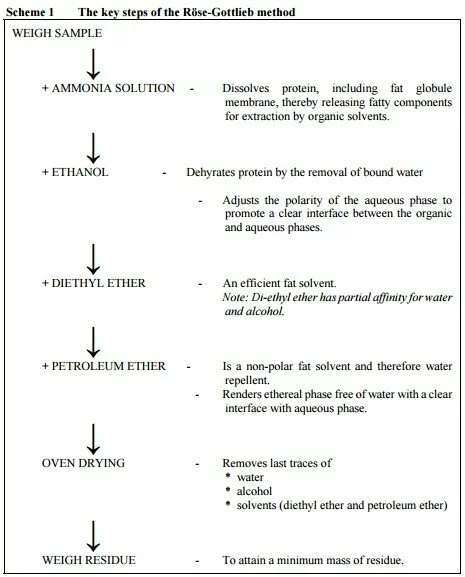
In other words: Total fat = sum of fatty acids from all sources, expressed as triglyceride. Hence only lipids that give fatty acids on hydrolysis plus free fatty acids are included in the definition. The analytical process needed is a complete extraction from the food material of fatty acids from all lipids containing fatty acids and free fatty acids, and subsequent gas chromatographic quantitation of individual fatty acids. Each individual fatty acid is mathematically converted to its triglyceride equivalent and these are summed to give the total fat content of the sample. The key steps of the Rˆse-Gottlieb method are shown in Scheme 1 on the next page.
Kjeldahl Method for Nitrogen Determination
The Kjeldahl method is the accepted reference method for the determination of protein in food, and was first published in 1883 by Kjeldahl, Head of Chemistry Department, Danish Brewing Company, Carlsberg, Denmark. The method is the basis for routine nitrogen assays and is used in many cases for the calibration of modern instruments used for rapid protein measurement, e.g. the “Milkoscan” for milk protein, and mid and near infrared instruments. The method involves the estimation of the total nitrogen in the food (it does not directly measure protein), and the conversion of the percentage nitrogen to protein, assuming that all the nitrogen in the food is present as protein, using a conversion factor based on the percentage nitrogen in the food protein.
% protein = % N x F F= conversion factor = 100/(%N in food protein) For dairy products, F = 100/15.68 = 6.38 For soya products, F = 5.71
Nitrogen Compounds In Dairy Products In dairy products, the nitrogen compounds are made up of 78% caseins, 17% whey proteins, which make up the protein N content, and 5% non-protein nitrogen (NPN), giving 100% total nitrogen (TN). The main constituents of the NPN fraction are urea (nearly 1/3), free amino acids (27%) and peptides (18%). Internationally and in New Zealand, dairy protein is measured as: Crude protein = TN x 6.38 However, true protein = (TN – NPN) x 6.38
Milk Proteins
A typical figure of protein content in milk is 3.5% (m/m). Proteins in milk exist two distinct groups; caseins and whey proteins.
Caseins make up 80% of milk proteins and are the integral components that make up the structure of the micelles of milk.
Whey proteins make up 20% of milk proteins and are present solubilised in the whey.
Caseins
- – Consist of alpha, beta, kappa and gamma caseins.
- – Contain phosphorus (0.8 g/100 g casein) and sulphur (0.74 g/100 g casein).
- – Coagulate at pH 4.6.
- – Affected by acid/alkalies.
Whey Proteins
- – Consist mainly of α-lactalbumin, β-lactoglobulin, globulins and albumin.
- – Whey proteins contain nitrogen (approximately 15.5%).
- – Contain sulphur (approximately 2½ times the amount in casein).
- – Contain no phosphorus.
- – Not affected by acid/alkalies.
The Chemical Steps Of The Kjeldahl Process
STAGE 1 – Digestion Stage Digestion of the sample with:
(a) concentrated H2SO4 (oxidising agent);
(b) sodium sulphate or potassium sulphate to raise the boiling point and speed up the reaction;
(c) catalyst (copper, titanium, selenium or mercury) to speed up the reaction. In the process, the nitrogen in the food (other than nitrate and nitrite nitrogen) is converted to ammonium sulphate.
N (food) → (NH4)2SO4
STAGE 2 – Distillation Stage
After the digest has been allowed to cool and diluted with water, the ammonium sulphate is then converted into ammonia gas by heating with sodium hydroxide.
2NaOH + (NH4)2SO4→ Na2SO4 + 2NH3 → + 2H2O
The ammonia is steam distilled into an excess of boric acid solution to trap the volatile ammonia by forming ammonium borate.
2NH3 + 2H3BO3→ 2NH4 H2BO3
Equation (A)
STAGE 3 – Titration Stage
The amount of borate (H2BO3 – ) formed is determined by titration with standard hydrochloric acid or sulphuric acid.
2NH4 H2BO3 + 2HCl → 2NH4Cl + 2H3BO3
Equation (B) or
2NH4 H2BO3 + H2SO4→ (NH4)2SO4 + 2H3BO3
Equation (C) Adding Equations (A) and (B) when using hydrochloric acid:
2NH3 + 2HCl → 2NH4Cl
Adding Equations (A) and (C) when using sulphuric acid:
2NH3 + H2SO4→ (NH4)2SO4
From the equations: 1
mole HCl = 1 mole N = 14 g N or 1 mL 0.1 M HCl = 0.0014 g N or 1 mole H2SO4 = 2 mole N = 28 g N or 1 mL 0.1 M H2SO4 = 0.0028 g N
The Kjeldahl method has advantages and disadvantages.
Advantages:
(a) It is used universally and is the standard method for comparison against other methods. (b) It has good precision and good reproducibility.
Disadvantages:
(a) The method is slow and uses strong oxidising acids (requires special safety precautions in laboratories) and the chemical waste from the test is not environmentally acceptable for normal sewer systems.
(b) It does not measure directly the protein content of a food. Instead total nitrogen (TN) is measured.
(c) Non-protein nitrogen (NPN) is included in the TN and this overestimates the protein content. Other instruments which provide rapid nitrogen analysis, based on the Dumas combustion method, have been recently introduced.
LACTOSE DETERMINATION
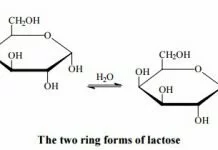
Lactose Lactose milk sugar is found only in the milk of mammals and is formed by a condensation reaction between carbon 1 of a galactose molecule and carbon 4 of a glucose molecule. Lactose is a reducing sugar, because the aldehyde group on the glucose molecule (C1) is not tied up in a glycosidic link.
Lactose and all monosaccharides and disaccharides are polar compounds, making them generally very soluble in water. This is due to the high number of OH groups present in the molecule. There are two crystalline forms (α and β) which differ in the arrangement of the groups at carbon as shown.
These forms differ in their optical rotation and, as for glucose, there is mutarotation which is rapid at high temperatures (> 75ºC) and slow at low temperatures.
![]()
The aldehyde form present in a solution of lactose explains why it is a reducing sugar. Lactose occurs in several crystalline forms, but only α-lactose monohydrate and β-lactose are important in dairy products. On mild oxidation, e.g. with alkaline copper (II), the aldehyde group is oxidised to a carboxylate group and copper(II) reduced to Cu(I):
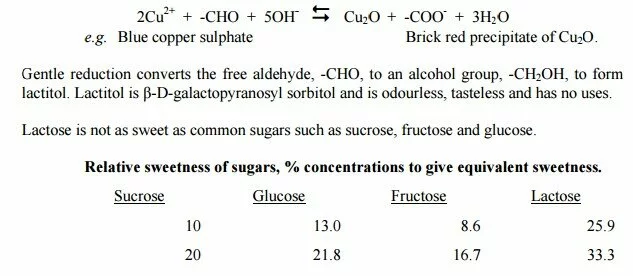
Lactose can be hydrolysed to D-glucose and D-galactose with mineral acid, cation exchange resins or enzymes (β-galactosidases). Like other sugars, lactose caramelises and decomposes when heated strongly in solution.
Lactose as a reducing sugar interacts with proteins and amino acids, particularly during the drying of dairy products. These processes are referred to as Maillard or browning reactions. These reactions are both desirable and undesirable in dairy products.
In some cases, the colour and Maillard flavour are desirable but there is also loss of nutritional value through destruction of the essential amino acid, lysine. A compound found in heated milk is lactulose, in which the glucose part of the lactose has been isomerised to fructose. Lactulose is somewhat sweeter and considerably more soluble than lactose.

TOTAL SOLIDS DETERMINATION
Definition Total solids (TS) represent the components that remain after the complete removal of water from milk. This is a rather ideal definition because it will be seen from the notes below that complete removal of free and bound water is probably not possible in most atmospheric drying methods.
Although it would appear to an inexperienced analyst that TS and moisture tests are very simple and easy to perform, and that results of good precision would be easy to obtain, this is not the case, because the more the TS test is examined in detail, a greater potential for errors is highlighted.
The problems in the TS test arise from the following and these factors often make it difficult to get good agreement within and between laboratories:
(a) After driving off moisture, the dried components are measured in the chemical state in which they are at that time, and the chemical state is dependent on the exact process or method the analyst has followed in the particular laboratory. (This emphasises the importance of following the method or standard exactly to ensure that good laboratory repeatability within laboratories and reproducibility between laboratories are obtained.)
(b) Sampling problems. Obtaining a representative sample from the milk is of utmost importance.
(c) Specific precautions that apply to all weight determinations, particularly very precise ones, are that fingerprints and air vapour have mass. In the TS method, once dishes are predried, they should be exposed to the atmosphere for a minimum amount of time and be handled only with tongs. All cooling should be in a desiccator.
(d) Quality control in the TS test is difficult because, as primary standards of known composition and similar matrix to milk are not available for testing, the procedure cannot be checked against standards.
(e) The performance of ovens needs to be checked to ensure that they meet the required prerequisites. The standard loading of the oven needs to be checked in each laboratory.
(f) There is the problem of removing volatile substances other than water by vaporisation, and also all bound water is never completely removed.
(g) Maillard browning can occur during drying, which results in decomposition of solid components and gives a lower TS test result. Also, oxidation can occur during extended heating, increasing weight, which may or may not be compensated for by the decrease in solids by Maillard browning. Many papers have been written on the comparisons of TS methods and these highlight many of the problems mentioned above.
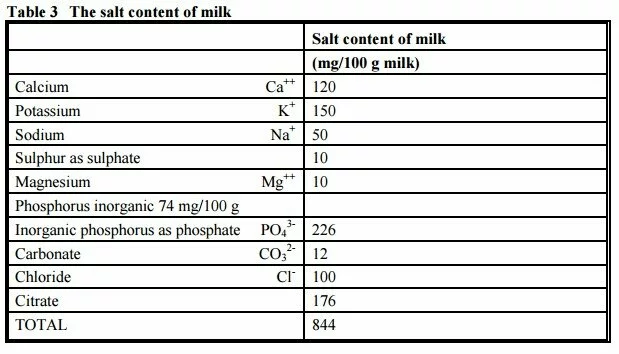
MINERALS
The table below shows the major minerals which are present in milk. There are many more present in trace amounts. These are analysed by a variety of techniques ranging from titrimetric for calcium, atomic absorption for magnesium, flame emission for sodium and potassium, colorimetric for phosphate, enzymatic bioanalysis for citric acid, etc.
Many of the trace elements can be analysed by newer techniques such as Inductively Coupled Plasma – Optical Emission Spectroscopy (ICP – OES) or Inductively Coupled Plasma – Mass Spectroscopy (ICP – MS).
SUMMARY
Thus as can be appreciated from the above, the dairy industry has come a long way from the days when all analyses were performed by traditional wet chemistry methods to the present where it is heavily involved with state of the art techniques and instrumentation. This represents the challenge for all analytical chemists.
Article written by C G Hughes and I K Gray, Food Science Section, New Zealand Dairy Research Institute.